Principles of Mammalian Cell Culture Process Scale‑up
Mammalian cell culture has developed over time to become the primary technology for the manufacture of monoclonal antibodies and recombinant protein therapeutics. Highly productive cell lines, high titers (5 to 10 g/L), established manufacturing platforms, development of chemically-defined media, and availability of bioreactors at a range of production scales are benefits of using this technology. However, the scale-up of cell culture processes from lab scale (1 to 10 L) to production scale (1 to 15 kL) remains a key challenge for many manufacturers. To successfully scale-up a process, numerous cell culture parameters, and the methods to control them across scales, must be considered, including pH, temperature, dissolved oxygen, carbon dioxide, osmolality, mixing times, gas transfer, and shear stress.
The BioProcess Technology Group within BDO has direct experience in cell culture scale-up and a broad perspective gained from helping numerous clients with this challenge. Here, we provide an overview of our approach to ensuring success when scaling up cell culture processes, the same principles of which can be applied when transferring processes between different types of bioreactors at the same manufacturing scale. The approach involves evaluating and optimizing bioreactor scaling parameters, process control, and gassing strategies.
Bioreactor Scaling Parameters
A key objective of any cell culture scale-up effort is to ensure that the process generates the desired product at a similar titer and of similar quality across scales. This is achieved primarily by ensuring that the cells experience similar environmental conditions (nutrients, pH, dissolved oxygen, carbon dioxide, osmolality profiles, shear stress) at each scale, while maintaining bioreactor homogeneity through mixing (agitation). In practice, this requires carefully determining the appropriate bioreactor operating conditions and process control strategies to provide the same environmental conditions for the cells at each scale.
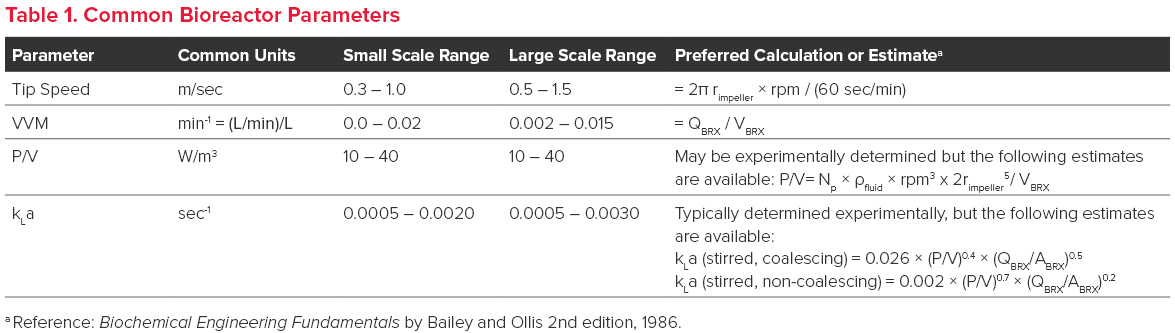
As bioreactor scale increases, agitation and gas sparging rates must also increase to ensure the maintenance of bioreactor homogeneity as well as dissolved oxygen and carbon dioxide concentrations. Traditionally, parameters such as impeller tip speed, gas flow rate (VVM), power per volume (P/V), and mass transfer coefficient (kLa) have been used to determine appropriate conditions for bioreactor mixing and gas sparging.
- Tip speed is, as its name implies, the speed of the impeller tip. This is the point of maximum shear created by the impeller and a potential locale for cell lysis to occur in the liquid phase.
- VVM is the gas flow rate per bioreactor volume. Generally, doubling the volume of the bioreactor requires doubling the gas flow.
- P/V (Power per volume) reflects the power per volume (Watts/m3) supplied to the culture through agitation. P/V is generally predictive of shear stress caused by agitation, and the agitation rate is usually set close to the maximum tolerable limit of P/V of the cells to ensure adequate mixing across scales, thereby avoiding pH and/or nutrient gradients.
- kLa reflects the dynamic response of a gas (O2 or CO2) to changes in bioreactor conditions (e.g., gas flow rate and agitation). kLa is either experimentally determined or fit to an empirical equation that is dependent on the P/V and gas flow rate (see Table 1.). Higher kLa values are generally required for cultures with high oxygen demand and can be achieved by increasing P/V or by micro-sparging.
Bioreactor Process Control
Controllers are used to maintain a favorable cellular environment in terms of temperature, pH, and dissolved oxygen, and these parameters should be controlled at the same set points across scales since all can impact cell growth, productivity, and product quality. Temperature is the easiest to control and tune because mammalian cell cultures do not generate or consume a significant amount of heat and only need to be warmed to physiological temperature (37°C). The culture pH is generally controlled by sparging CO2 gas into the culture to decrease pH, or by adding liquid base (sodium bicarbonate or sodium carbonate) to increase pH. To avoid excess addition of acid and base (which can lead to high osmolality), a pH dead-band is often used. Dissolved oxygen control generally employs a cascade control strategy where air sparging is used initially at low cell density, and oxygen is supplemented on demand as the cell density of the culture increases. Controller set-points and dead-band are consistent regardless of scale, but controller tuning (PID settings) must be tailored based on scale and equipment and are, ideally, independent of the specific cell culture process being scaled up.
Gassing Strategies
In lab-scale bioreactors, the gas-liquid surface area contributes significantly to gas transfer (O2 and CO2), but as the scale increases, this surface area becomes less significant relative to the culture volume; At larger scales, the gas-liquid surface area does not contribute significantly to gas transfer and sparging of air and oxygen directly to the culture is used to increase the total gas/liquid interface to enable greater gas transfer.
The challenge in developing a robust gassing strategy for scale-up is that gasses are sparged into the culture for multiple purposes: to maintain dissolved oxygen at a target set point, to control pH, and to remove excess CO2.
- To control dissolved oxygen, O2 gas is supplied to maintain a setpoint of 20 to 60% of air equivalent. Given the wide range of cell densities and bioreactor oxygen demand, it is common to employ a cascade control, sparging with only air initially then supplementing with oxygen in the sparge gas to maintain the oxygen set point.
- CO2 gas is generally used to decrease pH to maintain pH set point.
- Cells produce CO2 as part of normal metabolism and a common scaling difficulty is the buildup of CO2 in larger scale bioreactors. CO2 levels must be maintained at similar levels across scales to achieve similar process performance, and air sparging is commonly used to remove (or strip) CO2 from the bioreactor.
Providing all gases (air, O2, and CO2) through a single sparging line is problematic given one gas can impact more than one parameter and the requirements for each may conflict. For example, increasing air flow can strip CO2 but it can also reduce the oxygen concentration in the sparged gas, reducing the ability to control dissolved oxygen. Dual sparge (i.e., two sparge lines) can help overcome this limitation; oxygen can be sparged via a drilled-hole or micro-sparger while CO2 levels can be controlled with an open-pipe sparge with air. Oxygen sparging is often controlled with continuous monitoring and a feedback control loop, whereas carbon dioxide is often controlled via a preset aeration rate of air flow per bioreactor volume.
Another consideration when developing gassing strategies is the shear stress that mammalian cells experience at the gas/liquid interface as air bubbles burst. In order to mitigate this issue, surfactants such as Pluronic F68 (0.1%) can be added to the culture to prevent cells from sticking to bubbles. Additionally, antifoam is commonly added to reduce the amount of foam and bubbles at the surface.
Recommendations
To ensure successful cell culture process scale-up, care must be given to ensure that the cells experience similar environmental conditions across scales. Environmental conditions that can have an impact on cell growth, productivity and product quality include pH, temperature, dissolved oxygen concentration, carbon dioxide concentration, osmolality, and shear stress. The use of appropriate scaling parameters, bioreactor control strategies, and gassing strategies as described here will ensure successful scale-up.
SHARE